As the youngest of the coronavirus family, SARS-CoV-2 did his parents proud. Here's what we know about its genetic relatives and how it evolves by antigenic drift.
Viruses are not cells. They're not even technically alive, since they lack critical functions like growth and metabolism. But they do possess their own unique set of genes, allowing them to replicate, mutate, and evolve under the influence of our own biological equipment.
Types of Human Coronavirus
Billions of years of viral evolution has produced a stunning diversity of intracellular parasites. One particular group, called coronaviruses, thrive in mammals and birds; seven species of which have now broken through into humans.
All coronaviruses share the same fundamental features. Genetic material (stored as RNA) is protected by a nucleocapsid shell, which is further wrapped up in an envelope and studded with the now infamous spike proteins.
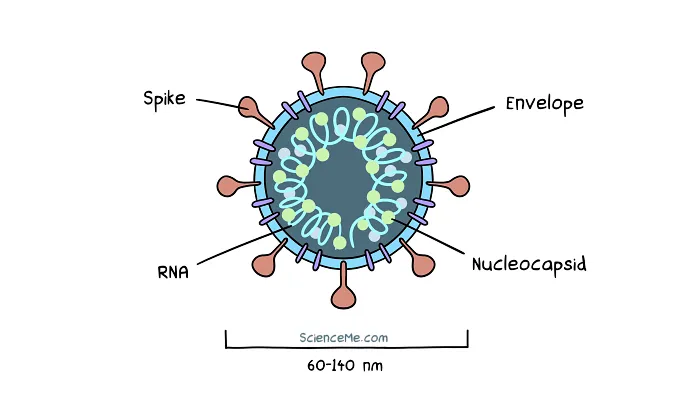
A cross-section of SARS-CoV-2, the virus that causes COVID-19.
Although there are only seven human coronavirus species, each of these can be broken down into countless variant subsets, differentiated by major mutations. Similarly, each variant can be broken down into multiple strains, brought about by minor mutations.
Most of us grew up with just three species of human coronaviruses: NL63, 229E, and OC43 cause common colds, and occasionally a spot of pneumonia.
But in recent years, no less than four new species have evolved: HKU1, MERS, SARS, and SARS-CoV-2. Unlike the long endemic coronaviruses, these new species hit the deck with much higher pathogenicity, causing a wider range of symptoms from a head cold, to brain damage, to total respiratory failure.
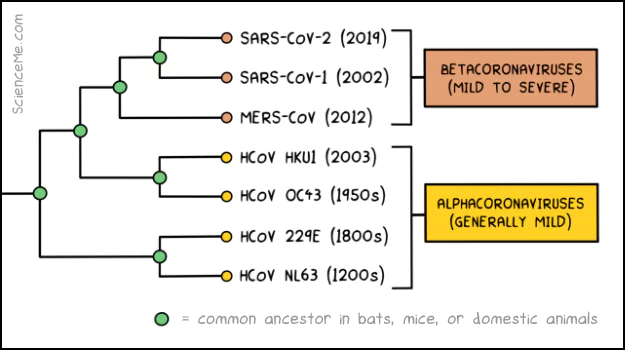
Genetic analysis and molecular clock dating give us the estimated date of origin of all seven coronaviruses: HCoV-NL63, HCoV-229E, HCoV-OC43, HCoV-HKU1, MERS-CoV, SARS-CoV-1, and SARS-CoV-2.
We're all exposed to endemic coronaviruses in the first few years of life. Fortunately, our young immune system is amped to tackle these onslaughts, rapidly destroying viral invaders and tucking their profiles away into long term memory—aka the adaptive immune system.
But as we age, our immune system becomes less responsive. Viral infections take an increasing toll on the body, and some can remain hidden in our tissues for years.
"RNA [of 229E] is detected in about 44% (40 of 90) of human brains tested." - Human Coronavirus (2021)
So when a novel virus hits humanity, the elderly tend to take a double hit. Not only is the immune system degraded, but it has zero experience of the new pathogen.
How Does COVID Compare to Other Diseases?
The latest Omicron variant is almost as infectious as measles, while being deadlier than seasonal flu and swine flu.
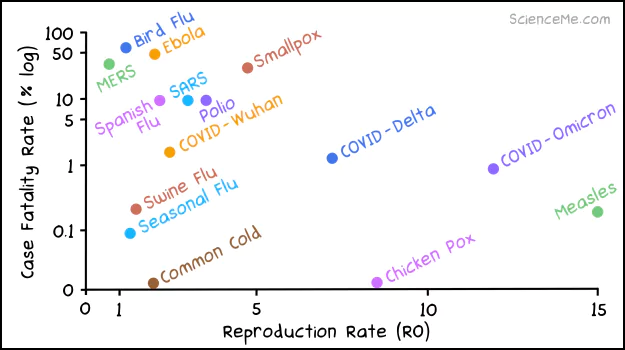
The fatality and infection rates of COVID variant compared to other infectious diseases.
Due to such widespread infection, SARS-CoV-2 continues to evolve rapidly, which gives COVID an evolving disease profile.
When the pandemic began, the average infected person spread the disease to 2.7 other people. This is known as the Reproductive Number (R0), and quadrupled in less than two years.
- Wuhan variant R0 = 2.7
- Delta variant R0 = 5.1
- Omicron variant R0 = 12
At the same time, the Case Fatality Rate (CFR) has fallen. This measures the rate of COVID infections that become fatal.
- Wuhan CFR = 1.6%
- Delta CFR = 1.3%
- Omicron CFR = 0.9%
However, while Omicron is less pathogenic than Delta, it's considerably more transmissible. This is how a milder variant can actually kill more people overall. To date, more than 1 million Americans have died from the pandemic.
How Do Viral Variants Evolve?
The evolution of SARS-CoV-2 comes down to the spike protein: the biological key that allows the virus to gain entry to our cells.
The spike protein contains a critical cluster of amino acids known as the Receptor Binding Domain. When a spike makes contact with a cell receptor, the RBD oscillates, jiggling the key in the lock. The receptor binds to the virus and draws it into its membrane.
At the same time, the spike protein shapeshifts. The prefusion spike folds and breaks apart, taking on a new molecular configuration known as the postfusion state.
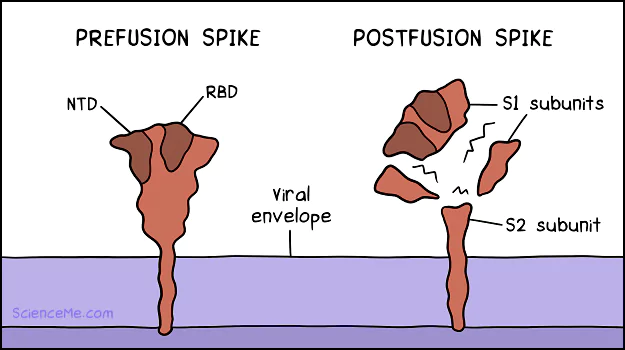
The shapeshifting spike protein. (1) In the prefusion state, the RBD oscillates to unlock ACE2 cell receptors. Likewise, the NTD can unlock AXL cell receptors. (2) On binding, the spike protein is cleaved into postfusion subunits: S1 subunits float free, while the S2 subunit remains anchored.
However, targeting a single antigen puts all our eggs in one basket. Whether we're infected naturally or vaccinated against SARS-CoV-2, our antibodies are geared strongly toward the spike protein.
This creates an evolutionary pressure on the virus to evolve different shaped spike proteins. Natural selection favours the spikes that bind most efficiently while being harder to recognise by the immune system.
And viruses evolve fast. Every time a virus replicates inside a cell, it has the opportunity to mutate. In fact, mutations are so common that we can trace the genetic signature and link individual COVID cases to relatively small clusters of infections.
Mutations in the SARS-CoV-2 virus have changed the structure of the spike protein. This is antigenic drift.
How has antigenic drift played out in the evolution of SARS-CoV-2?
- Delta gained two RBD mutations, allowing the viral variant to bind faster, leading to accelerated viral replication. The Delta variant also had affinity for lung tissue which is loaded with ACE2 receptors.
- Omicron gained 15 RBD mutations, allowing the variant to replicate almost 100x faster than Delta. The Omicron variant targets the upper respiratory tract which increases transmission.
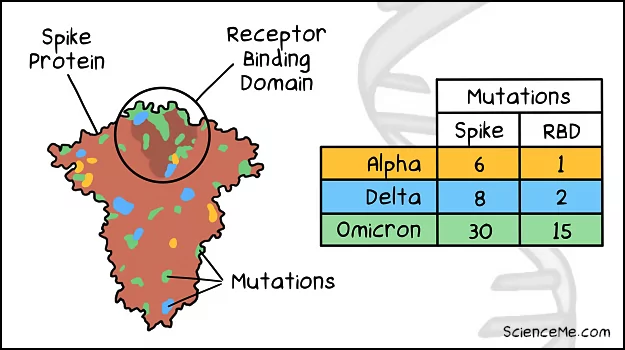
Comparing the spike mutations of the Omicron, Delta, and Alpha variants.
How Will The Pandemic End?
The proliferation of Omicron is hoped to give us better herd immunity against SARS-CoV-2. But even when the pandemic ends, COVID is unlikely to go away; endemicity simply means the end of exponential spread, with infection rates falling to a predictable background rate.
We're not out of the woods yet. And it's possible that a deadlier SARS-CoV-2 variant will emerge. The Spanish Flu, which killed an estimated 50 million people, evolved to be deadlier before it decreased in pathogenicity, while Ebola has become more pathogenic over time.
History suggests COVID will be with us for the rest of our lives. It's shaping up to be a highly infectious disease that's worse than flu and mutates rapidly, necessitating annual vaccinations. In the meantime, scientists and politicians can only guess as to the best strategic moves as complex pandemic dynamics play out.
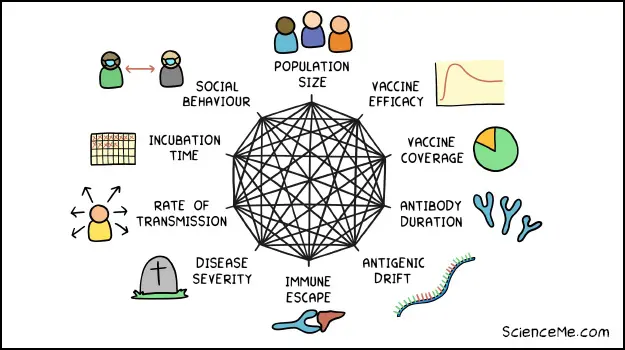
Major drivers of pandemic dynamics.
Written and illustrated by Becky Casale. If you like this article, please share it with your friends. If you don't like it, why not torment your enemies by sharing it with them? While you're at it, subscribe to my email list and I'll send more science articles to your future self.